
Practices that facilitate the safe administration of COVID-19 vaccines
The Institute for Safe Medication Practices (ISMP) recently published a bulletin sharing a number of leading practices that facilitate the safe administration of a COVID-19 vaccine. These practices, which incorporate learning from past vaccine errors, will evolve over time as we gain additional knowledge.
In Canada, at the time ISMP published its bulletin, Health Canada had approved two COVID-19 vaccines (known by the names of their manufacturers: Moderna and Pfizer-BioNTech), both of which use mRNA technology.
Since the release of the bulletin on February 25th, two more vaccines have been approved in Canada, the Oxford Astra-Zeneca and the one-shot Johnson&Johnson vaccines. Both vaccines are easier to transport and store than the earlier approved vaccines.
The ISMP document looks at two groups of vaccine errors — worldwide incidents reported thus far involving COVID-19 vaccines and errors that occurred in Canada with other vaccines.
Storage
Each COVID-19 vaccine has distinct storage requirements, and all vaccines approved so far require storage in a refrigerator, freezer, or ultra-low-temperature freezer. An interruption in the cold chain could result in product spoilage. For example, several incidents have been traced to inadequate equipment for monitoring the temperature of freezers and refrigerators, and/or the lack of a plan to deal with temperature excursions. To limit potential wastage of vaccine, process checks must be in place to ensure public health guidance, as well as manufacturers’ temperature storage requirements, are followed.
Vaccine storage, whether in a refrigerator or freezer, must be carefully scrutinized to ensure products are appropriately segregated. Errors involving selection of the wrong product have occurred when refrigerators were poorly organized. For example, there have been instances when insulin, a neuromuscular blocker or another vaccine was administered instead of the intended vaccine product. In all of these incidents, a key contributing factor was storage of multiple products in close proximity. Ideally, bar-coding will be used during the vaccine selection step, as well as during preparation and administration.
The Pfizer-BioNTech product requires dilution after thawing, but the diluent is not supplied with the product and must be sourced separately. Inoculation of undiluted vaccine has been reported. Bundling the diluent vial with the vaccine vial when the latter is removed from the freezer provides workflow support to help prevent errors.
Preparation
Develop a standardized process for preparing doses and provide a quick-reference sheet at the point of dose preparation. Errors related to vaccine preparation include withdrawal and administration of the contents of an entire multidose vial to a single patient; needle-syringe misconnection; preparation of an incorrect volume; as well as inadequate or no dilution, and administration of diluent only. To prevent preparation errors, United States Pharmacopeia (USP) developed a COVID-19 Vaccine Handling Toolkit highlighting a number of operational considerations.
As part of the standardized process, labelling of prefilled syringes is essential because it provides an opportunity for an extra check before dose administration. The syringe label should specify the name of the product (and its manufacturer), the dose (volume) for injection, the lot number, and the beyond-use date and time (i.e., the last date and time of day when the product can be used). It is also important to label any opened, partially used vials with both the puncture and beyond-use dates and times.
Administration
The person administering the vaccine should be alert to visual cues indicating a potential undetected error from a previous step (e.g., appearance of syringe contents differing from expectations, incorrect volume). Injection of an incorrect volume for multiple patients has been reported. Review of a labelled syringe prior to administration supports the identification of a potential error.
Before administration of a COVID-19 vaccine, each patient should be screened for contraindications or reasons that would affect their suitability for vaccination. For example, current guidelines advise separating administration of the COVID-19 vaccine by 14-28 days from most other vaccine products (e.g., shingles vaccine). If possible, the timing of future inoculations with a COVID-19 and other planned vaccines needs to be considered.
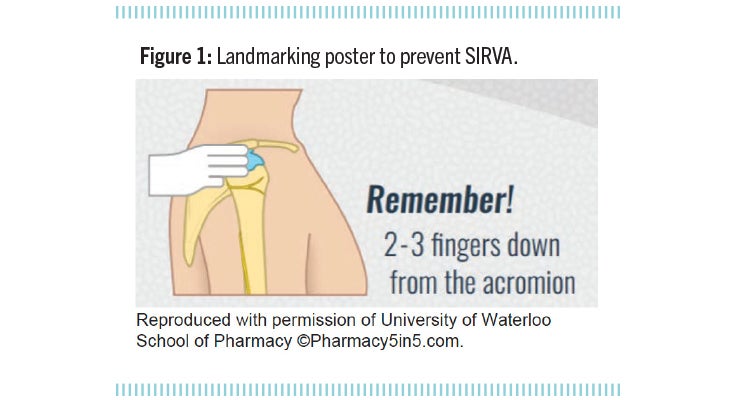
Shoulder injury related to vaccine administration, also known as SIRVA, is a well-established preventable adverse effect resulting from improper landmarking technique (Figure 1). It is easier for those administering the vaccine to identify the correct injection site if the patient’s upper arm is bare. Accordingly, patients should be advised to wear suitable clothing (i.e., sleeveless/short-sleeved tops) to their appointment. In addition, vaccine stations should be set up to allow privacy.
Documentation
Three of the four approved vaccines require two doses for maximum efficacy — there is a risk that the wrong product could be used for the second dose. At the time of publication, the National Advisory Committee on Immunization (NACI) is recommending the vaccine series for each person be completed with the same COVID-19 vaccine; however, NACI also acknowledges that if the same product is not available at the time of the second dose, a vaccine of similar type (e.g., another mRNA product, if the first dose was an mRNA product) can be used.
Providing patients with documentation of vaccination can prevent errors. When patients presenting for their second dose do not know which product they received initially, such documentation can be used to confirm or identify the correct vaccine to be administered. Patients can also be encouraged to take a picture of the documentation or to enter it into a medication app such as MyMedRec or CanImmunize.
Documentation also supports the prevention of scheduling errors. Moderna and Pfizer have their own recommended intervals between the two required doses. Information about the COVID-19 vaccines provided by Health Canada and NACI includes information about the timing of doses, as well as the timing of these doses in relation to administration of other vaccine products.
Booking a patient’s second appointment at the time of the first visit will support vaccination at the right time, when possible. There has been a report describing a scheduling error due to miscommunication. Follow-up by phone, text, or email is recommended to ensure patients receive the second dose on time.
Conclusion
Knowledge about the appropriate use and safety of COVID-19 vaccines will continue to evolve, and guidance from health authorities, such as Health Canada, NACI, public health organizations, and provincial/territorial health ministries, will be updated regularly.
Vaccine-related adverse drug reactions should be reported through the customary reporting channels. In addition, consider documenting vaccine errors (regardless of outcome) through an organization’s usual reporting process and/or ISMP Canada’s Individual Practitioner Reporting program. These reports will support future learning and error prevention.
Summary
- Storage: Be ready to respond to temperature excursions (e.g., refrigerator failure) to prevent waste.
- Preparation: Provide a quick-reference sheet at the point of dose preparation, and label all prefilled syringes.
- Administration: Use a checklist to identify vaccine contraindications or precautions.
- Documentation: Provide patients with documentation of vaccination. MyMedRec and CanImmunize apps are options to store this information.
Figure 1 transcript
Landmarking poster to prevent SIRVA
Remember! 2-3 fingers down from acromion
Reproduced with permission of University of Waterloo School of Pharmacy ©Pharmacy5in5.com












